- Table of Contents
1. What is Medical Translation and Pharmaceutical Translation?
Human Science, which provides translation services in various fields such as IT, is also a translation company that deals with the medical sector, while simultaneously being a technology company that develops and operates various translation-related technologies, offering a wide range of solutions.
https://www.science.co.jp/localization/industry/medical/index.html
In this column, we will discuss medical and pharmaceutical translation and the key points for selecting a translation service provider.
Medical Translation Target
On the introduction page for Human Science's medical and medical translation services, we present the translation targets in the medical industry, divided into "Pharmaceutical and Medical Translation Services" and "Medical Device Translation Services."
https://www.science.co.jp/localization/industry/medical/index.html
One of the most important aspects of "pharmaceutical and medical translation" is the translation of documents created by pharmaceutical companies, such as those related to clinical trials and safety reports to regulatory authorities (including reports on efficacy and pharmacology studies, pharmacokinetics studies, toxicity studies, safety studies, clinical trial drug summaries, clinical trial protocols, informed consent documents, clinical trial summary reports, case reports, etc.).
In addition, we provide Japanese translations of papers and conference materials published in English medical journals, as well as academic medical translations of papers written by Japanese physicians and English translations of diagnostic and treatment guidelines created by Japanese medical societies.
Medical device translation includes not only documents created during development, manufacturing, and approval, but also the translation of various manuals such as instruction manuals and catalogs.
In addition, in recent years, there has been an increase in demand in the field of biotechnology (such as cell analysis, genetic analysis, and the equipment and lab supplies for these purposes).
Additionally, as a new trend in the production materials of medical-related companies, there are internal training materials and video content that incorporate animations and videos.
Demand and Future Potential of Medical Translation
In recent years, the medical industry has gained significant attention due to the COVID-19 pandemic. In fact, PCR testing equipment, vaccine development, ECMO, and ventilators have become major topics of discussion. So, what is the actual market situation? It seems to vary depending on whether we are looking at the pharmaceutical industry or the medical device industry, and whether we are considering the domestic market or the global market. However, as a whole, the medical industry appears to have relatively stable demand, which tends to be less affected by economic fluctuations, and the demand for translation services also seems to be stable.
In the medical field, the demand for translations related to clinical trials is increasing year by year.
As international clinical trials are on the rise and multilingual support is required, accurate translations are essential.
Documents such as clinical trial protocols and informed consent forms are representative examples of the documents that need to be translated, and the accuracy of translations for clinical trial documents is crucial as it directly impacts the success of the trials.
The Difference Between Medical Translation and Other Translations
There are several important differences between medical translation and other types of translation.
- Requires advanced and specialized knowledge
First of all, a significant characteristic of medical translation is that it requires advanced and specialized knowledge. The medical field has many unique terminologies and abbreviations, and the ability to accurately understand and appropriately translate them is essential. For example, without specialized knowledge, mistranslations can easily occur with drug names, disease names, treatment methods, and diagnostic names, potentially having serious impacts on patient health.
Furthermore, since medical knowledge is updated daily, it is necessary to constantly acquire the latest medical knowledge from academic conferences and research papers to perform medical translation.
On the other hand, in general translation, it is rare for specific specialized knowledge to be essential; instead, the ability to understand context and cultural background and to replace expressions appropriately is emphasized. Of course, specialized knowledge may be required in other fields such as technical translation or legal document translation, but it is often not as demanding as in medical translation.
・Accuracy and consistency are required
In medical translation, accuracy and consistency are particularly important. Medical documents often have legal aspects, and mistranslations can lead to legal issues. Therefore, translators must always update their medical knowledge and maintain terminology consistency. In contrast, general translation often requires flexibility in style and expression, and creative approaches may be allowed.
- Ethical considerations are necessary
Furthermore, ethical considerations are also important in medical translation. Confidentiality to protect patient privacy and clear, concise expressions to avoid misunderstandings with patients are required. In contrast, general translation often does not demand the same level of confidentiality or ethical considerations.
In summary, medical translation is a highly specialized field that requires advanced expertise, accuracy, consistency, and ethical considerations, necessitating a different skill set and sense of responsibility compared to other types of translation.
Is a qualification necessary for medical translation?
When it comes to qualifications in the medical field, one might first think of the qualifications for doctors or pharmacists. However, it is not realistic for current professional translators or those aspiring to become professional translators to obtain the aforementioned qualifications, especially a medical license. Therefore, to demonstrate practical translation skills, I would like to introduce the JTF Translation Certification, which includes the medical and pharmaceutical fields in its certification exams. Levels 1 and 2 are considered challenging, with a pass rate below 5%, and can be seen as an official proof of practical translation skills in the medical and pharmaceutical fields.
In addition to the JTF Translation Certification, there are qualifications that prove medical translation abilities.
・JTA Certified Translation Professional Qualification
・Japan Medical English Proficiency Test (IMEP)
・Clinical Trial Practical English Test
・Translation Practical Examination TQE
・Amelia Regular Trial
Examples include.
However, there are many people who, even without qualifications, have gained experience in translation based on their educational background in fields such as medicine, pharmacy, biology, and biochemistry, as well as their work experience in pharmaceutical companies, CROs, medical device manufacturers, medical publishers, and medical advertising agencies.
In actual translation work, it is not always the case that you are assigned projects that perfectly match the fields studied for certification preparation. When carrying out tasks, adherence to various rules such as client specifications, familiarity with designated translation tools, and a high level of speed and accuracy are required. These aspects often speak more to practical experience and a proactive attitude than to the possession of qualifications.
■Required Skills for Medical Translation
Language Proficiency
The language proficiency required for medical translation is not significantly different from that of other practical translations, so we will omit the explanation regarding the level of English proficiency. However, here we will introduce the uniqueness of English in medical translation.
As an example of English translation, when submitting an English paper to an overseas medical journal, one must be well-versed in the structure of the paper and unique expressions. Additionally, each journal has submission guidelines that specify the style and format in detail. There may be a tendency among Japanese people to think, "If the content is good, they might overlook some deviations in style..." However, in reality, deviations in style can be a bottleneck and may prevent passing the peer review.
In the case of Japanese translations, not only medical papers mentioned above but also documents related to clinical trials, safety reports, and other pharmaceutical-related documents each have their own unique formats, styles, and expressions, and translations that adhere to these are required.
On the other hand, for newsletters, white papers, and catalogs of medical devices, it is necessary to accurately translate texts that incorporate marketing expressions while maintaining a high level of expertise.
Additionally, for subtitles and dubbing translations in videos of doctors' lectures, roundtable discussions, and training for doctors or within companies, it is essential to convey the main messages spoken, including specialized content, without omitting any information, while also reducing the number of characters and making the text easier to hear and read.
Expert Knowledge
While there is a high level of expertise in general practical translation, the specialized knowledge required for medical translation is extensive and profound.
Here, I will specifically list each medical field below.
Neurology Area Cardiovascular Area Respiratory Area Gastroenterology Area Gynecology Area Urology Area Dermatology Area Dentistry Area Ophthalmology Area Orthopedics Area Surgery Area Psychiatry Area Endocrinology and Metabolism Area Cancer Area Anesthesiology Area
In each of these areas, new drugs and medical devices are developed, academic societies engage in activities such as formulating diagnostic guidelines, physicians perform medical practices and submit papers, and there are various literature, books, and academic journals.
All documents generated from these are highly specialized.
When it comes to translation, specific knowledge and skills are required. For pharmaceutical translations, knowledge of clinical trials is necessary. For medical paper translations, familiarity with medical knowledge and academic papers is essential. In the case of medical device translations, knowledge of the relevant medical field is required, as well as familiarity with application-related documents, manufacturing procedures, and user manuals. Depending on the type of medical device, knowledge in mechanics, optics, chemistry, and physics may also be required. Additionally, foundational knowledge in medicine, pharmacy, biology, biochemistry, and statistics is common.
However, it is naturally extremely difficult to master all of these at once, and even highly skilled translators typically build their experience and know-how in a specific field, such as cardiology, before venturing into new areas.
2. The Importance of Medical Translation and Pharmaceutical Translation
The situation has changed due to the COVID-19 pandemic since early 2020, but the trend of increasing foreign visitors and foreign workers in Japan over the past few years before the pandemic is expected to remain a significant long-term trend. It is not difficult to imagine that having medical information provided in one's native language, such as in hospitals and for pharmaceuticals, would be beneficial for those living in or visiting Japan. This also highlights the importance of medical translation.
However, there are many other important reasons for medical translation and pharmaceutical translation.
■Handling essential matters related to life and health, not trends
Healthcare institutions, pharmaceutical manufacturers, CROs, medical device manufacturers, and sales companies for drugs and medical devices all take pride in handling essential matters related to life and health, not trends, in their daily work. It goes without saying that the documents generated in the medical and pharmaceutical fields are important. Translating these documents quickly and accurately to deliver them to those in need is a highly meaningful task.
■Contributing to the International Introduction of Pharmaceuticals
In the development of pharmaceuticals, the concept of "international joint development" has become common. According to the Ministry of Health, Labour and Welfare's document on "International Joint Clinical Trials ( https://www.mhlw.go.jp/shingi/2007/07/dl/s0727-11d_0002.pdf )", it states that "clinical trials planned for the global development and approval of new drugs, in which multiple medical institutions from different countries or regions participate in a single trial, and proceed simultaneously based on a common clinical trial protocol. Primarily, Phase III trials are targeted." In short, the concept aims to eliminate the disparities in the timing of new drug introductions among countries by conducting the development, trials, and approvals of new drugs simultaneously worldwide.
As someone involved in translation, by quickly and accurately translating documents that arise at each stage of new drug development, clinical trials, and approval (from Japanese to English for Japanese pharmaceutical companies, and from English to Japanese for overseas), we can contribute to global healthcare.
It is undoubtedly important to contribute to the rapid introduction of useful medical devices, whether from domestic to overseas or from overseas to domestic.
Global simultaneous product development and launch is common in IT-related localization and consumer goods, but the rapid introduction of pharmaceuticals and medical devices related to life and health seems to have a different level of importance.
3. The Challenges of Medical Translation and Points to Note
By reading up to this point about the skills required for pharmaceutical translation and the importance of medical and pharmaceutical translation, I believe you have understood that high language proficiency and specialized knowledge are necessary, and that due to the importance of medical translation, accuracy and speed are required.
Let us provide some examples of the challenges and considerations in medical translation.
In medical books and medical papers published by publishers, it is common to request supervision and editing from university professors or authoritative doctors in the relevant field. Additionally, it is often the case that pharmaceutical companies act as sponsors.
Medical books and papers require meticulous translation, and among the supervisors and editorial committee members responsible for proofreading, there are many who demand an exceptionally high level of translation. Furthermore, since the supervisors and editorial committee members are usually extremely busy, those involved in the translation must ensure the highest quality before presenting it for their review. Additionally, during the production process, the sponsoring pharmaceutical companies are also involved in verifying the translation, and in the case of pharmaceutical companies, there is a strong tendency to be very careful about whether the effectiveness and safety of the drugs mentioned in the content (regardless of whether they are their own products or those of competitors) are accurately described.
In the safety departments of pharmaceutical companies and CROs, it is necessary to quickly report information regarding adverse events of collected pharmaceuticals, including the name of the adverse event, severity, and causal relationship, to the authorities. The reporting deadlines to the authorities are determined by the severity of the event, causal relationship, and other factors.
Although the case reports used for safety reporting, such as CIOMS, are often considered relatively straightforward in terms of writing difficulty, they are still unique documents with a high level of specialization. Additionally, for drugs that are currently undergoing clinical trials, it is not uncommon for a large volume of documents to be generated at once, requiring a high level of promptness.
Furthermore, factors such as administration to pregnant women, administration to infants, administration outside of specified indications, and administration exceeding the specified dosage are also very important elements when reporting to the authorities. Therefore, if there are errors in the translation that could affect the determination of these factors, it could lead to issues. Thus, it is essential for safety personnel at pharmaceutical companies and CROs to ensure accurate translations to avoid incorrect judgments regarding severity and causal relationships.
Here, let's take a look at actual text from clinical trial documents.
- (English) Study aaaaaaa is a randomized study evaluating bbbbbbb monotherapy versus physician’s choice (ccccccc, ddddddd, or eeeeeee) for the treatment of mCRPC patients with a BRCA1/2 or ATM mutation who have received prior treatment with AR-directed therapy but have not yet received fffffff chemotherapy in the castration-resistant setting.
- (Example translation) This is a randomized trial to evaluate the efficacy of this drug as a monotherapy in mCRPC patients with BRCA1/2 mutations or ATM mutations who have a history of androgen receptor (AR) targeted therapy but have not received chemotherapy, compared to other agents selected by the physician (ccccccc, ddddddd, or eeeeeee).
If we were to use machine translation to translate the above text, current machine translation could produce a translation of a certain level. Moreover, the ability to generate translations instantly and in large quantities is a significant advantage.
However, if the sentence is of a certain length as mentioned above, it is necessary to check whether there are any issues with the structure of the translation, and it is also essential to confirm whether the technical terms frequently included in the text are translated appropriately (ensuring they are not general expressions, that they are the most contextually suitable terms, and that there is no inconsistency in the translations).
In the case of human translation, if the translator is excellent, they will consider and research not only the elements mentioned above but also what the main subject of the text, the investigational drug, is, what the purpose of the trial mentioned in the text is, and what impact the trial results have on the drug's clinical trial while translating.
However, machine translation does not make judgments like the above; it merely analyzes the text and refers to the corpus. Therefore, confirming the content mentioned above needs to be done carefully in the post-editing process.
Translation clients and translation companies should consider these factors and appropriately determine whether to use machine translation or human translation, taking into account the required translation quality level, deadlines, and budget. It seems necessary to examine how to proceed with the translation project. Additionally, if there are many reused sections in existing documents due to revisions or other reasons when using human translation, utilizing translation support tools like Trados to leverage existing translations as translation memory becomes a viable option.
4. Key Points for Selecting a Medical Translation Service
Finally, we will introduce the key points to consider when selecting a provider for medical translation services.
A Company with Highly Specialized Translators
Translating medical documents presents challenges as discussed in this column. As a fundamental requirement, it is essential to have a translation company with a wealth of highly specialized and experienced pharmaceutical and medical translators.
A company with a translation department specializing in medical translation
A translation company with a proven track record with various medical-related companies and institutions both domestically and internationally provides a sense of security. It may be a good idea to check their website to confirm their achievements.
A company that can provide technology-equipped solutions
In medical translation, where large volumes of documents must be translated quickly, it is becoming increasingly difficult to exclude the use of machine translation and translation assistance tools from the outset. Additionally, new technologies such as videos and e-learning content are continuously emerging. It can be said that a company that has a proven track record in machine translation and translation assistance tools, and can also accommodate new content, is desirable for providing a wide range of solutions.
5. Introduction of Medical Translation Cases
Full review system by 150 medical and pharmaceutical translators and specialized reviewers
At Human Science, we believe that one of the key factors affecting the quality of translation is the review (check) process after the translation work is completed. Therefore, we have a system in place where experienced professional reviewers (checkers) thoroughly check the entire translation.
This process allows us to detect and appropriately correct errors and mistakes made during the translator's work stage. Additionally, from a professional standpoint, it enhances the accuracy of the translation and contributes to the stabilization of overall translation quality by coordinating translations from multiple translators.
Pharmaceutical and Medical Translators
We have 150 experienced translators in the medical and clinical trial fields. We provide checks and English proofreading by native speakers with M.D. (Doctor of Medicine) and Ph.D. (Doctor of Philosophy) degrees.
![]()
Japanese-English Translator
Ph.D
- Areas of Expertise
- Bio, Genetics, Computational Chemistry
- Achievements
- Oncology (Protocol), Gynecology (ICF), Cell Biology (Paper)
![]()
Japanese-English Translator
26 years of experience in pharmaceutical translation. 15 years working at a pharmaceutical translation company.
- Areas of Expertise
- Oncology
- Achievements
- Lung cancer (CSR), colorectal cancer (IB), PMDA inquiry items, clinical trial protocol, clinical trial summary report, case report form, informed consent document, investigational drug summary, PMS, inquiry items
![]()
Japanese-English Translator
25 Years of Experience in Pharmaceutical Translation
- Areas of Expertise
- Regenerative Medicine (iPS Cells)
- Achievements
- Cardiology (papers), Leukemia (papers), Respiratory field (clinical guidelines)
![]()
English-Japanese Translator
12 Years of Experience in Pharmaceutical Translation
- Areas of Expertise
- Diabetes, Hemophilia
- Achievements
- Colorectal cancer (thesis), breast cancer (summary report), diabetes (thesis)
![]()
Japanese-English Translator
10 years of experience working at a pharmaceutical research institute
- Areas of Expertise
- Oncology, CMC
- Achievements
- Biopharmaceuticals (CTD 2.3), Oncology (Review Report), CMC
![]()
Japanese-English Translator
Pharmacist
- Areas of Expertise
- Cardiology, Oncology
- Achievements
- Urothelial Carcinoma (Protocol), Antibody Drugs (CTD 2.3), Inferior Vena Cava Filter (CSR)
![]()
English-Japanese
24 years of translation experience
10 years working at a pharmaceutical translation company
- Areas of Expertise
- Clinical, Non-Clinical, Post-Marketing Surveillance
- Achievements
- Clinical trial protocol, clinical trial summary report, investigational drug brochure, informed consent document, toxicity tests, pharmacokinetic tests, PMDA inquiry items, package insert
![]()
English-Japanese
24 years of translation experience
10 years working at a pharmaceutical translation company
- Areas of Expertise
- Clinical, Non-Clinical, Post-Marketing Surveillance
- Achievements
- Clinical trial protocol, clinical trial summary report, investigational drug brochure, informed consent document, toxicity tests, pharmacokinetic tests, PMDA inquiry items, package insert
English Proofreading
14 Years of Proofreading Experience
8 Years in Research Positions at Overseas and National Universities
- Areas of Expertise
- Clinical, Non-Clinical, Post-Marketing Surveillance
- Achievements
- Explanatory consent documents, case reports, clinical trial protocols, SOPs, submission papers, various diagnostic guidelines
![]()
Japanese-English
5 years of translation experience
4 years in research positions
- Areas of Expertise
- Clinical, Non-Clinical
- Achievements
- Case reports, clinical trial protocols, informed consent documents, investigational drug brochures, conference materials, medical literature, academic papers
English Proofreading / Doctoral Review
4 Years of Proofreading Experience
PMDA Advisor, Clinical Fellow at National Research Institute, Licensed Physician
- Areas of Expertise
- Clinical, Non-Clinical, Post-Marketing Surveillance
- Achievements
- Ministry of Health, Labour and Welfare notifications, PMDA inquiry items, safety reports, submission papers, various diagnostic guidelines, press releases, various internal documents
Medical Device Translator
To provide high-quality translations, translators with prior knowledge of diagnostic and therapeutic procedures involving specific medical devices will be responsible for the translations.
15 years of translation experience
15 years of experience working for a domestic medical device manufacturer
Medical Device
Academic papers, clinical trial protocols, test equipment specifications, clinical trial summary reports, technical reports, user manuals, defect reports, documents for submission to government agencies
3 years of translation experience
Experience in a foreign pharmaceutical company
11 years of work experience
Pharmaceuticals, Medical Devices
User manuals, software-related documents, attachments, investigational drug brochures, clinical trial protocols, reports for exhibitions/conferences, academic papers
13 years of translation experience
Experience in a domestic pharmaceutical company
8 years of work experience
Pharmaceuticals, Medical Devices
Clinical trial protocol, investigational drug brochure, clinical trial summary report, informed consent document, letter to the principal investigator, pharmaceutical package insert, adverse event report
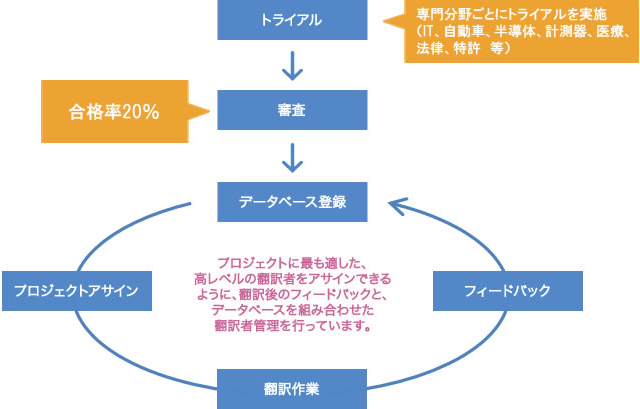
Hiring translators and post-editors who meet the quality standard with a pass rate of 20%
Human Science has 150 translators and 75 post-editors, and we conduct selection and trial assessments of translators and post-editors specialized in the industry.
Only translators and post-editors who have passed the review by native translation directors, considering their track record, are assigned to projects.
We have established strict standards, with a pass rate of 20% for translators and 10% for post-editors.
With an average experience of over 5 years, we guarantee reliable quality.
Here are some examples of medical translation
We have a track record of transactions with various companies, from pharmaceutical companies to medical device manufacturers.
- Pharmaceutical Company
- ASKA Pharmaceutical Co., Ltd.
- Otsuka Pharmaceutical Co., Ltd.
- Mitsubishi Tanabe Pharma Corporation
- Chugai Pharmaceutical Co., Ltd.
- Novartis Pharma K.K.
- Ryukakusan Co., Ltd.
- CRO
- ICON plc, Japan
- Quintiles Transnational Japan, Inc.
- Shin Nihon Kagaku PPD Co., Ltd.
- Parexel International Corporation
- Mediscience Planning Inc.
- DOT World Inc.
- Medical Device
- AREI INC.
- Illumina, Inc.
- Carl Zeiss Meditec AG
- Konica Minolta, Inc.
- Seed Corporation
- NIDEK CO., LTD.
- NIPPON KODEN KOGYO CO.,LTD.
- Becton, Dickinson and Company Japan
- Medtronic Japan Co., Ltd.
- Baxter International Inc.
- Fujifilm Medical Co., Ltd.
- Beckman Coulter, Inc.
- Boston Scientific Japan K.K.
- Rion Co., Ltd.
- Life Sciences Analytical Instruments
- Illumina, Inc.
- Sartorius Japan K.K.
- Cosmic Corporation
- Thermo Fisher Scientific Inc.
- Thermo Fisher Diagnostics Inc.
- Japan Waters Corporation
- General Incorporated Association Japan Analytical Instruments Manufacturers' Association
- Life Technologies Japan, Inc.
- Levity Japan, Inc.
- STEMCELL Technologies
- Japan Tissue Engineering Co., Ltd.
- Others
- Japanese Respiratory Society
- Japanese Society of Allergology
- National Center of Neurology and Psychiatry
- Medical Review Company, Inc.
- Jihou Co., Ltd.
- Kurecon Research & Consulting Co., Ltd.
- Ensis Corporation
- TARGIS CO.,LTD.
- CareNet Inc.
- ArisGlobal
■Leave Medical Translation to Human Science
Human Science, which has accumulated achievements and know-how in translation support tools, machine translation, and checking tools, has provided high-quality translations in the medical field, where expertise is required, through a combination of human and technology, ensuring short delivery times. Please take advantage of Human Science's services that offer high precision, speed, and cost efficiency, along with the reassurance of work done by excellent professionals.