Medical Device Translation Services
- Localization: HOME
- Search by Industry
- Medical Translation Top
- Medical Device Translation Services
Systematically accumulating knowledge and know-how of various medical devices.
Translators with prior knowledge are in charge.
In the field of medical devices that support various medical settings both domestically and internationally, there is a wide range of products for both diagnosis and treatment, and understanding these products cannot simply be categorized under the single term "medical devices."
At Human Science, while translating and localizing instructions for use (IFU), various manuals, and other related documents for medical devices with unique characteristics, we have systematically accumulated knowledge and know-how. By utilizing translation support tools like Trados and checking tools like HS XChecker, we provide translation services that enhance efficiency and accuracy.
Track Record
- Supported Products
- Stents and catheters (PTCA balloons, IABP balloons, for angiography, central venous, Foley, and various other types and locations) and related products
- Pacemakers, defibrillators (ICD, etc.) and related products
- Assistive artificial heart, artificial heart valves / artificial lungs, artificial lung membranes, and related products
- Artificial kidneys, blood circuits, dialysis membranes, and dialysis-related products
- Artificial joints, artificial bones, bone fixation devices, and related products
- Intraocular lenses, contact lenses, and related products
- Therapeutic devices such as radiation therapy devices, laser therapy devices, ultrasound therapy devices, and related products
- Imaging diagnostic equipment (MRI, CT, PET, SPECT, etc.) and DICOM-related products
- Medical Imaging Diagnosis System
- Ophthalmic Medical Devices
- Testing equipment and instruments for immunoserological tests, biochemical tests, blood tests, urine tests, etc., and in vitro diagnostic reagents
- Testing equipment for respiratory and circulatory function tests, ultrasound tests, electroencephalogram tests, etc.
- Others (endoscopes, laparoscopes, artificial blood vessels, artificial skin, stone crushing devices, medical cleaning and sterilization equipment for medical facilities, hearing aids, home medical devices, etc.)
- Supported Formats
- Instruction Manuals (IFU: Instructions for Use), Operation Manuals, Various Manuals
- Software, UI
- Application Notes, Spec Sheets
- Catalogs, news releases, websites, white papers, and other promotional materials
- In-house training materials, and more
Our Features
We also accommodate requests such as the following.
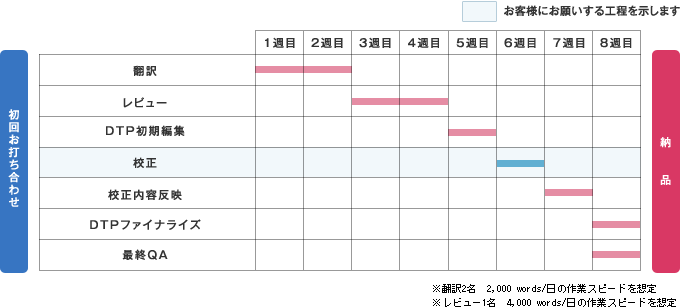
Schedule and Delivery Date
Human Science's medical device translation services also meet the demands of customers seeking speed.
As an example, if a new manual of 200 pages (equivalent to 40,000 words) is created, the estimated number of working days is as follows.
With our ability to support each process meticulously and the latest care backed by an academic background, we will fully back up your Japanese or translation into various languages.
Non-Disclosure Agreement and Security Measures

If you have any requests, we have a non-disclosure agreement in place when reviewing data or reference materials, even for quote requests or trial translations. We physically limit access to the data and reference materials we receive to specific workers, and each worker is required to strictly adhere to information handling compliance and submit a pledge or similar document.
Additionally, we conduct virus checks and take measures to ensure that no security issues arise with the emails and data we send, so you can rest assured.
Furthermore, if you provide your security standards, we can also submit the specified response documents or reports from our side.
Case Studies
- Nidek Corporation Ophthalmic Medical Device User Manual Multilingual Translation and Machine Translation Implementation
- Translation Volume: 1 million words per year × 5 languages
- Achieve cost reduction and shortened duration for manual translation with the introduction of machine translation
Case study on the introduction of machine translation at Nidec Corporation, click here for details - Domestic Ophthalmic Medical Device Manufacturer Clinical Trial Report English-Japanese Translation
- Translation Volume: 1,200 pages, 230,000 words
- Delivery Time / Work Period: 1.5 months
- Utilizing Trados to compress translation time. By conducting translation work and updating translation memories in parallel, we achieve shorter delivery times and consistency in translations.
- User Manual English-Japanese Translation for Foreign Medical Device Manufacturers
- Translation Volume: 222 pages, 35,000 words
- Delivery Time / Work Period: 2 months
- Revision of manuals due to system upgrades.
- There is a need for consistency in terminology and expressions with the old version, utilizing Trados.
- Foreign-affiliated medical device manufacturer - Mammography device - User manual - English-Japanese translation
- Translation Volume: Approximately 2,000 pages
- Delivery Time / Work Period: Approximately 1 year (Regular translation work)
- Clinical Medical Support System Company, Medical Imaging Diagnosis System, Mammography Dedicated Image Viewer
Software specifications, user manuals, UI, FDA application documents, Japanese-English translation - Translation Volume: Approximately 1.4 million characters
- Delivery Time / Work Period: Approximately 1 year (Regular translation work)
- Utilize Trados to proceed with the translation of multiple items in parallel
- We provide not only translation but also consulting for manual creation








