Features
Reliable quality management system and
overwhelming cost performance
At Human Science, we have established a unique quality control system based on various know-how cultivated through our past experiences, which we provide to our customers.
There are solid reasons for being chosen in the field where expertise and reliability in healthcare are highly valued.
Cost Reduction with Machine Translation Tools and Post-Editing

Human Science provides translation services centered around machine translation to reduce translation costs.
However, machine translation alone cannot meet the quality standards required in specialized fields such as healthcare and medical devices.
Therefore, post-editors with prior knowledge of the diagnostic and therapeutic practices related to the medical devices being translated ensure translation quality.
This system allows us to offer cost-effective translation services.
For details on the machine translation tool Mtrans, click here
For details on post-editing services, click here
Shorter delivery times with translation support tools Trados/Phrase TMS
In the pharmaceutical and medical fields, there are often cases where multiple translations of similar content occur in succession, or where a large volume of translations needs to be completed in a short period of time. In such cases, it is necessary to deploy multiple translators simultaneously and proceed with the work while coordinating with each other at the operational level.
Our company has established a system where dedicated contact personnel appropriately manage multiple translators and operate projects while maintaining quality levels. We utilize translation support tools such as Trados, Phrase TMS, and MemoQ.
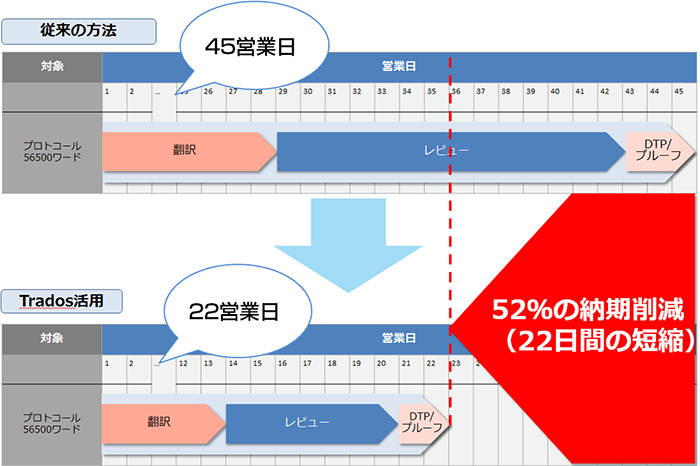
Examples of Reduced Delivery Times
Clinical Trial Protocol 56,000 Words English to Japanese Translation
- For example, in the case of a 56,000-word English to Japanese translation, if done manually by one translator and one reviewer without using translation support tools
- ・Translation: 28 business days
・Review: 13 business days
・DTP Proof: 3 business days
Total 45 business days - When using Trados/Phrase TMS
- ・Translation: 13 business days
・Review: 6 business days
・DTP Proof: 2 business days
Total of 22 business days with a 52% reduction in delivery time
Thorough Quality Control
1. Full review by a specialized reviewer
At Human Science, we believe that one of the key factors affecting the quality of translation is the review (check) process after the translation work is completed. Therefore, we have a system in place where experienced professional reviewers (checkers) thoroughly check the entire translation.
This process allows us to detect and appropriately correct errors and mistakes made during the translator's work stage. Additionally, from a professional standpoint, it enhances the accuracy of the translation and contributes to the stabilization of overall translation quality by mutually adjusting translations from multiple translators.
Pharmaceutical and Medical Translators
We have 150 experienced translators in the medical and clinical trial fields. We provide checks and English proofreading by native speakers with M.D. (Doctor of Medicine) and Ph.D. (Doctor of Philosophy) degrees.
![]()
Japanese-English Translator
Ph.D
- Areas of Expertise
- Bio, Genetics, Computational Chemistry
- Achievements
- Oncology (Protocol), Gynecology (ICF), Cell Biology (Paper)
![]()
Japanese-English Translator
26 years of experience in pharmaceutical translation. 15 years working at a pharmaceutical translation company.
- Areas of Expertise
- Oncology
- Achievements
- Lung cancer (CSR), colorectal cancer (IB), PMDA inquiry items, clinical trial protocol, clinical trial summary report, case report form, informed consent document, investigational drug summary, PMS, inquiry items
![]()
Japanese-English Translator
25 Years of Experience in Pharmaceutical Translation
- Areas of Expertise
- Regenerative Medicine (iPS Cells)
- Achievements
- Cardiology (papers), Leukemia (papers), Respiratory field (clinical guidelines)
![]()
English-Japanese Translator
12 Years of Experience in Pharmaceutical Translation
- Areas of Expertise
- Diabetes, Hemophilia
- Achievements
- Colorectal cancer (thesis), breast cancer (summary report), diabetes (thesis)
![]()
Japanese-English Translator
10 years of experience working at a pharmaceutical research institute
- Areas of Expertise
- Oncology, CMC
- Achievements
- Biopharmaceuticals (CTD 2.3), Oncology (Review Report), CMC
![]()
Japanese-English Translator
Pharmacist
- Areas of Expertise
- Cardiology, Oncology
- Achievements
- Urothelial Carcinoma (Protocol), Antibody Drugs (CTD 2.3), Inferior Vena Cava Filter (CSR)
![]()
English-Japanese
24 years of translation experience
10 years working at a pharmaceutical translation company
- Areas of Expertise
- Clinical, Non-Clinical, Post-Marketing Surveillance
- Achievements
- Clinical trial protocol, clinical trial summary report, investigational drug brochure, informed consent document, toxicity tests, pharmacokinetic tests, PMDA inquiry items, package insert
![]()
English-Japanese
24 years of translation experience
10 years working at a pharmaceutical translation company
- Areas of Expertise
- Clinical, Non-Clinical, Post-Marketing Surveillance
- Achievements
- Clinical trial protocol, clinical trial summary report, investigational drug brochure, informed consent document, toxicity tests, pharmacokinetic tests, PMDA inquiry items, package insert
English Proofreading
14 Years of Proofreading Experience
8 Years in Research Positions at Overseas and National Universities
- Areas of Expertise
- Clinical, Non-Clinical, Post-Marketing Surveillance
- Achievements
- Explanatory consent documents, case reports, clinical trial protocols, SOPs, submission papers, various diagnostic guidelines
![]()
Japanese-English
5 years of translation experience
4 years in research positions
- Areas of Expertise
- Clinical, Non-Clinical
- Achievements
- Case reports, clinical trial protocols, informed consent documents, investigational drug brochures, conference materials, medical literature, academic papers
English Proofreading / Doctoral Review
4 Years of Proofreading Experience
PMDA Advisor, Clinical Fellow at National Research Institute, Licensed Physician
- Areas of Expertise
- Clinical, Non-Clinical, Post-Marketing Surveillance
- Achievements
- Ministry of Health, Labour and Welfare notifications, PMDA inquiry items, safety reports, submission papers, various diagnostic guidelines, press releases, various internal documents
Medical Device Translator
To provide high-quality translations, translators with prior knowledge of diagnostic and therapeutic procedures involving specific medical devices will be responsible for the translations.
15 years of translation experience
15 years of experience working for a domestic medical device manufacturer
Medical Device
Academic papers, clinical trial protocols, test equipment specifications, clinical trial summary reports, technical reports, user manuals, defect reports, documents for submission to government agencies
3 years of translation experience
Experience in a foreign pharmaceutical company
11 years of work experience
Pharmaceuticals, Medical Devices
User manuals, software-related documents, attachments, investigational drug brochures, clinical trial protocols, reports for exhibitions/conferences, academic papers
13 years of translation experience
Experience in a domestic pharmaceutical company
8 years of work experience
Pharmaceuticals, Medical Devices
Clinical trial protocol, investigational drug brochure, clinical trial summary report, informed consent document, letter to the principal investigator, pharmaceutical package insert, adverse event report
2. Utilize our in-house developed XChecker to streamline terminology and style checks
At Human Science, we use our uniquely developed checking tool, XChecker, to quickly verify compliance with style guides and glossaries.
Supports various file formats and can detect errors that are difficult for the human eye to catch, such as numerical inaccuracies and inconsistencies in translations, leading to a significant reduction in work time.
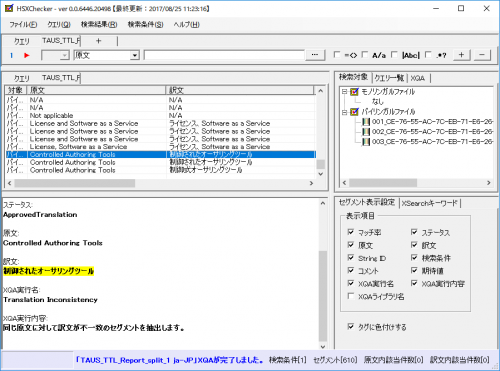
HS XChecker Screen
Training and Management of Translators and Post-Editors
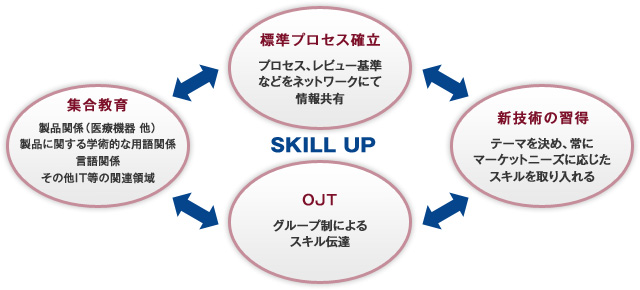
We have established specialized teams such as the "Standardization Promotion Committee" and the "Technical Promotion Committee" within the company, working to enhance the individual skills of our staff for quality management and improvement, as well as to establish and enforce standard processes.
1. Recruitment of translators and post-editors who meet the quality standard with a pass rate of 20%
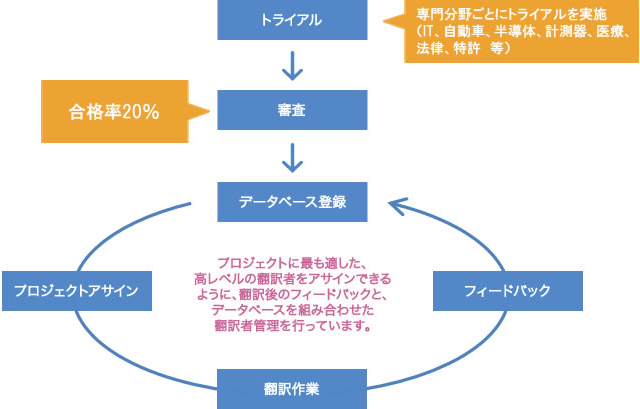
Human Science has 150 translators and 75 post-editors, and we conduct selection and trial assessments of translators and post-editors specialized in the industry.
Only translators and post-editors who have passed the review by native translation directors, considering their track record, are assigned to projects.
We have established strict standards, with a pass rate of 20% for translators and 10% for post-editors.
With an average experience of over 5 years, we guarantee reliable quality.
2. Centralized Management System for Translators through Database Integration
We have established a system to provide feedback on the internal review results to all translators after the completion of work.
During feedback, we not only provide evaluation scores but also point out specific areas to the translators and present examples of corrections to promote continuous quality improvement.
Additionally, by centralizing the above feedback and the performance and evaluations of translators (evaluations from clients and internal assessments) in a database, we have created a system to select the most suitable translators.