On May 27, 2020 (Wednesday), we co-hosted a seminar titled "Shortening Lead Times for Medical Translation! Utilizing Machine Translation/Memsource" with Memsource.
We introduced the use of machine translation and translation support tools as a means to achieve shorter delivery times for pharmaceutical companies and CROs involved in medical and pharmaceutical translation.
We will introduce the cost reduction rate and post-editing in machine translation, which have received positive feedback.
- Table of Contents
1. Cost Reduction Rate in Machine Translation
The top reasons for introducing machine translation are "shortening delivery times" and "reducing costs."
While it is possible to achieve "shortened delivery times" and "cost reduction" by using machine translation without making any corrections,
Documents translated by machine translation cannot be released to the market or submitted to authorities without checking and corrections.
However, if too much time is spent on correcting machine-translated text, or post-editing, the goals of "shortening delivery times" and "reducing costs" will not be achieved, leading to a situation that defeats the purpose.
One reason why the implementation of machine translation does not proceed smoothly is the difficulty in establishing a workflow related to post-editing after machine translation.
However, by establishing quality standards and an overall workflow for machine translation, we can reduce the workload by about half for certain translation items.
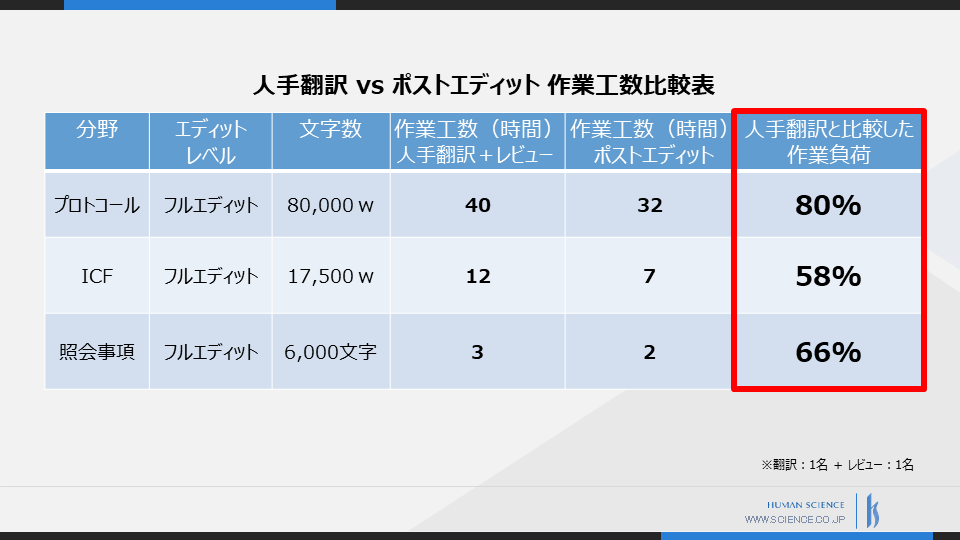
The following is a comparison table of the hours spent on post-editing machine translation of protocols, ICFs, and inquiries, compared to human translation.
By establishing quality standards and an overall workflow for machine translation, we have been able to reduce the workload by over 20%, and nearly 50% in the case of ICF.
In addition to comparing time, how about cost and amount?
The following is a graph of cost reduction in a project that incurred costs of 100 million yen due to human translation.
This is a case study that successfully achieved a cost reduction of 40 million yen. Due to the large scale of the project, an initial investment of 10 million yen was required, but even after accounting for that, it is clear that there was a significant cost reduction effect.
So, specifically, how should we proceed with post-editing?
2. Quality Standards and Post-Editing Levels
In this article, we define post-editing as the process of comparing the source text with the output of machine translation to check whether the required quality of the translated text is met and making corrections as necessary.
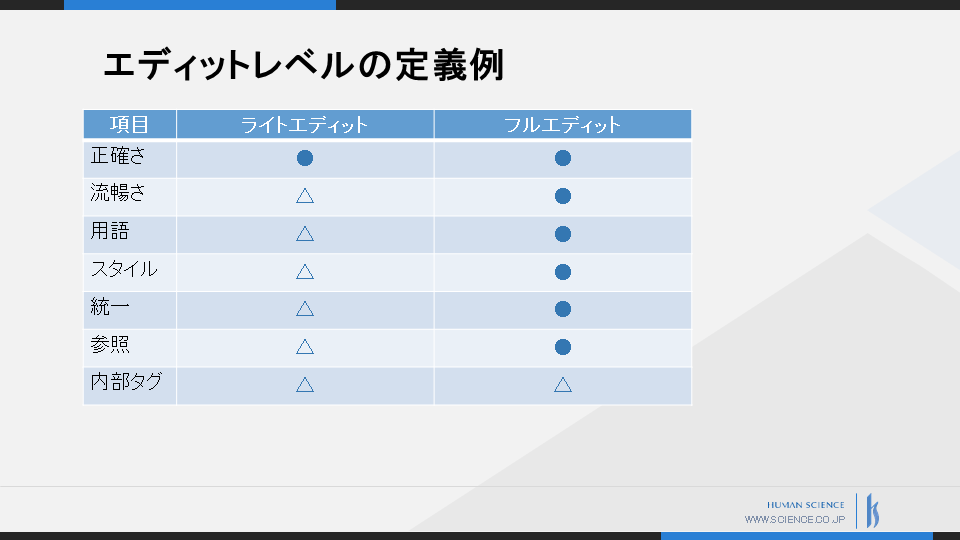
At Human Science, we have established seven quality criteria for post-editing, including accuracy and fluency.
We categorize the level of editing into 'light edit' and 'full edit', and define the editing items to be implemented according to the target document.
Before starting the project, we agree with the client on the quality level we aim for and then begin the work.
3. Example Sentences for Post-Editing in Medical and Pharmaceutical Translation
The following is an example of post-edited translated documents in the field of medical and pharmaceutical translation.
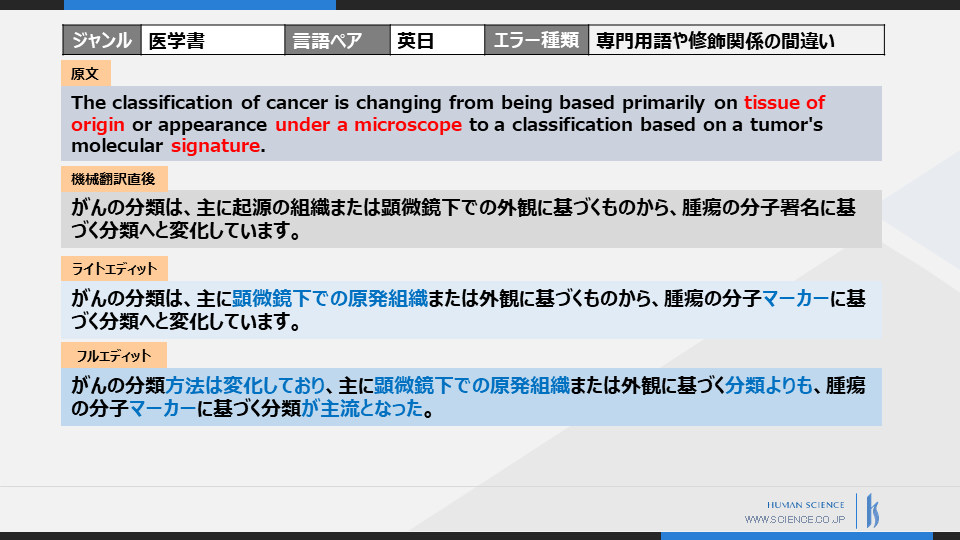
Light edit is a correction from the perspective of "accuracy".
Two mistranslations have been corrected.
First, since the modification relationship of "on tissue of...a microscope" is different, it should be corrected to "primary tissue under a microscope or..."
The second point is the mistranslation of technical terms. Since 'signature' is incorrectly translated as 'signature', it has been corrected to 'marker'.
Light Edit guarantees accuracy, so corrections stop here.
Full edit now includes fluency as a criterion, adjusting the text to be more natural.
While "cancer is changing" is translated as "is changing" in machine translation, it is improved in quality by being translated as "is changing... and has become mainstream."
You can download additional example sentences from below. Please take a look if you like.
4. Documents suitable for machine translation
Machine translation has been shown to have a significant impact on efficiency and cost reduction.
However, it is not necessarily the case that any document can be efficiently translated using machine translation.
While there are areas of expertise with MT engines, below are some that are generally suitable for machine translation.
.png)
Documents with a strong internal material flavor are relatively suitable for MT. However, documents submitted to PMDA, FDA, and EMA require accuracy, so it cannot yet be said that MT is their strong suit. Exceptionally, ICFs written in plain language can be considered suitable for MT.
Rather than categorizing it as machine translation, it is necessary to consider it based on the type and purpose of the document.
I have briefly introduced the content discussed in the seminar.
In addition, we plan to hold a web seminar with Memsource again in September 2020, covering the same content.
If you would like to receive seminar notification emails, please register using the button below.